It blew up! The FDA was defeated in the case! Pfizer was compelled to reveal vaccine data! There are 9 pages of possible side effects! The entire network was taken aback...
Pfizer lost court case and required to disclose all the serious side effects.

But yesterday, the latest news shocked many people!
Pfizer Vaccine was forced to release real vaccine data. Among them , a 9-page report on "Pfizer Vaccine Adverse Reactions" instantly exploded on Twitter!
I am text block. Click edit button to change this text. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.

That's exactly what happened:
The US Food and Drug Administration FDA lost the lawsuit on March 1. The FDA was ordered by the court to complete the disclosure of 329,000 pages of complete review documents for Pfizer's new crown vaccine by the end of the summer. The FDA has now released the first set of documents.
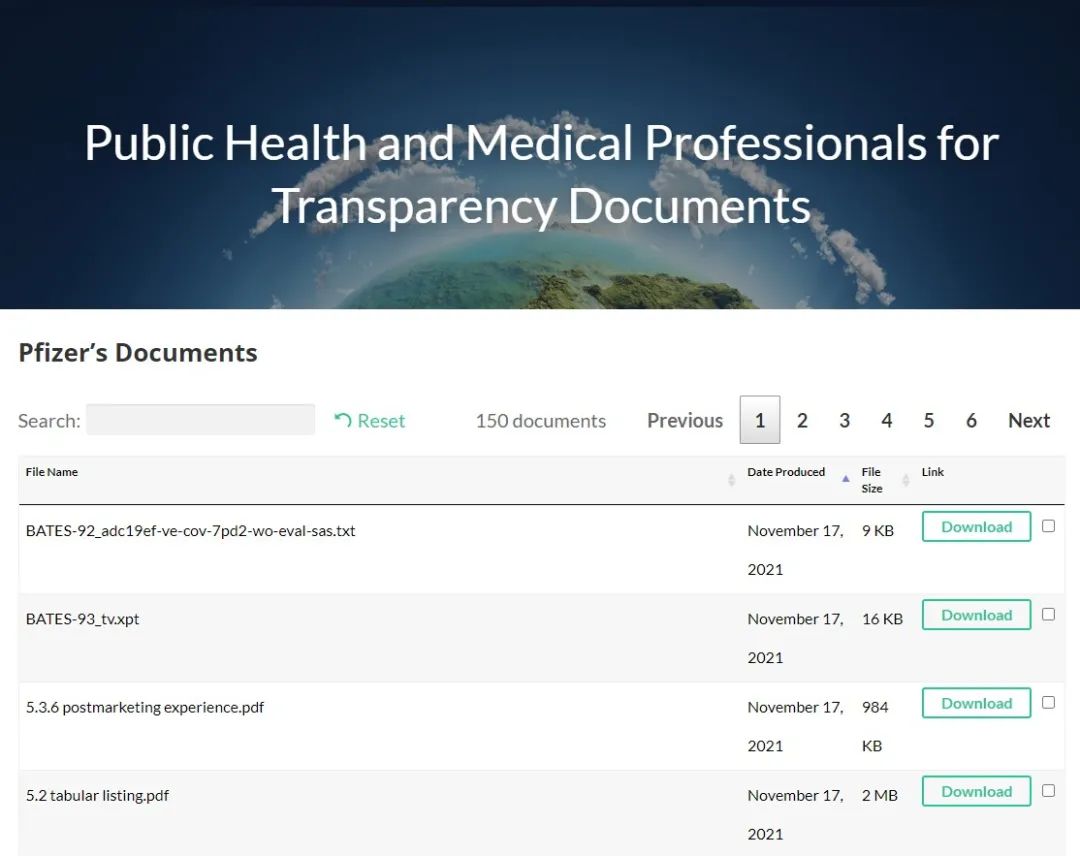
However, no interpretation or organisation of the document is provided. On its website, 150 documents are currently available for free download.
The process of this case, on the other hand, is full of twists and turns.
Transparency Public Health and Medical Professionals Group (PHMPT), a nonprofit, is taking the FDA to court. Last September, it filed a lawsuit against the FDA in the United States District Court for the Northern District of Texas. The FDA's decision to withhold data on Pfizer and BioNTech's 2019 COVID-19 vaccine is being questioned.
Transparency Public Health and Medical Professionals Group (PHMPT), a nonprofit, is taking the FDA to court. Last September, it filed a lawsuit against the FDA in the United States District Court for the Northern District of Texas. The FDA's decision to withhold data on Pfizer and BioNTech's 2019 COVID-19 vaccine is being questioned.
The PHMPT organisation requested that the FDA fully disclose the vaccine review data in November of last year, but the FDA did not respond.
Following that, the PHMPT organisation filed a lawsuit against the FDA.
However, the FDA asked a federal judge to limit the disclosure of relevant documents to 500 pages per month. In other words, until 2076, it will take 55 years to fully disclose the 329,000 pages of information!
The PHMPT organisation was enraged: many of those who created, approved, and vaccinated had already died by 2076.
The plaintiff believes that the FDA violated US federal law, which states that "once the licence is obtained, the data and information of the biological product during the approval process can be immediately publicly disclosed," which is consistent with the FDA's commitment to "complete transparency" before and after the approval of the new crown vaccine.
The PHMPT organisation was enraged: many of those who created, approved, and vaccinated had already died by 2076.
The plaintiff believes that the FDA violated US federal law, which states that "once the licence is obtained, the data and information of the biological product during the approval process can be immediately publicly disclosed," which is consistent with the FDA's commitment to "complete transparency" before and after the approval of the new crown vaccine.
This week saw significant progress as a result of the lawsuit. The FDA lost the case in Texas District Court on March 1. Beginning that day, a large number of review documents concerning the "safety and effectiveness" of the Pfizer vaccine must be released one after the other.
The report on the adverse reaction of the Pfizer vaccine, which has 9 pages, is the most shocking among the many documents, and many netizens exploded in an instant!

Fasciitis, ocular swelling, facial paralysis, alopecia areata, anaphylactic shock, pregnancy anaphylaxis syndrome, acute cardiomyopathy, acute respiratory failure, injection site vasculitis, epilepsy, thrombosis, arrhythmia, arthritis, asthma, bronchospasm, cardiac arrest Sudden cardiac arrest, heart failure, chest discomfort, asphyxia, acute cutaneous lupus erythematosus, acute encephalomyelitis, acute kidney injury, acute outer macular retinopathy, aplastic anaemia, chronic autoimmune glomerulonephritis, chronic spontaneous urticaria, hemolytic anaemia, colitis, dermatitis, diabetes, embolic cerebral infarction, endocrine disorders, neonatal myasth
Link to more information: http://phmpt.org/wp-content/uploads/2021/11/5.3.6-postmarketing-experience.pdf
According to relevant reports, among the released documents, the document labelled as a priority review request contained more than 100 pages of anonymous safety-related data sheets, and some other documents also included gender, age, and BMI tables of unidentified participants; some typical drug or vaccine application documents; a document demonstrating that Pfizer paid the FDA nearly $2.9 million in standard user fees; and Fast Track designation letters (which are typically not released).
When this matter became public, it immediately sparked heated debate among Twitter users:
"In the Pfizer trial, 42,000 of the 46,000 participants experienced adverse effects, and 1,200 died. According to FDA regulations, if one person dies within 30 days of receiving an experimental investigational drug, the trial must be terminated. But it never happened, and we need to figure out why."